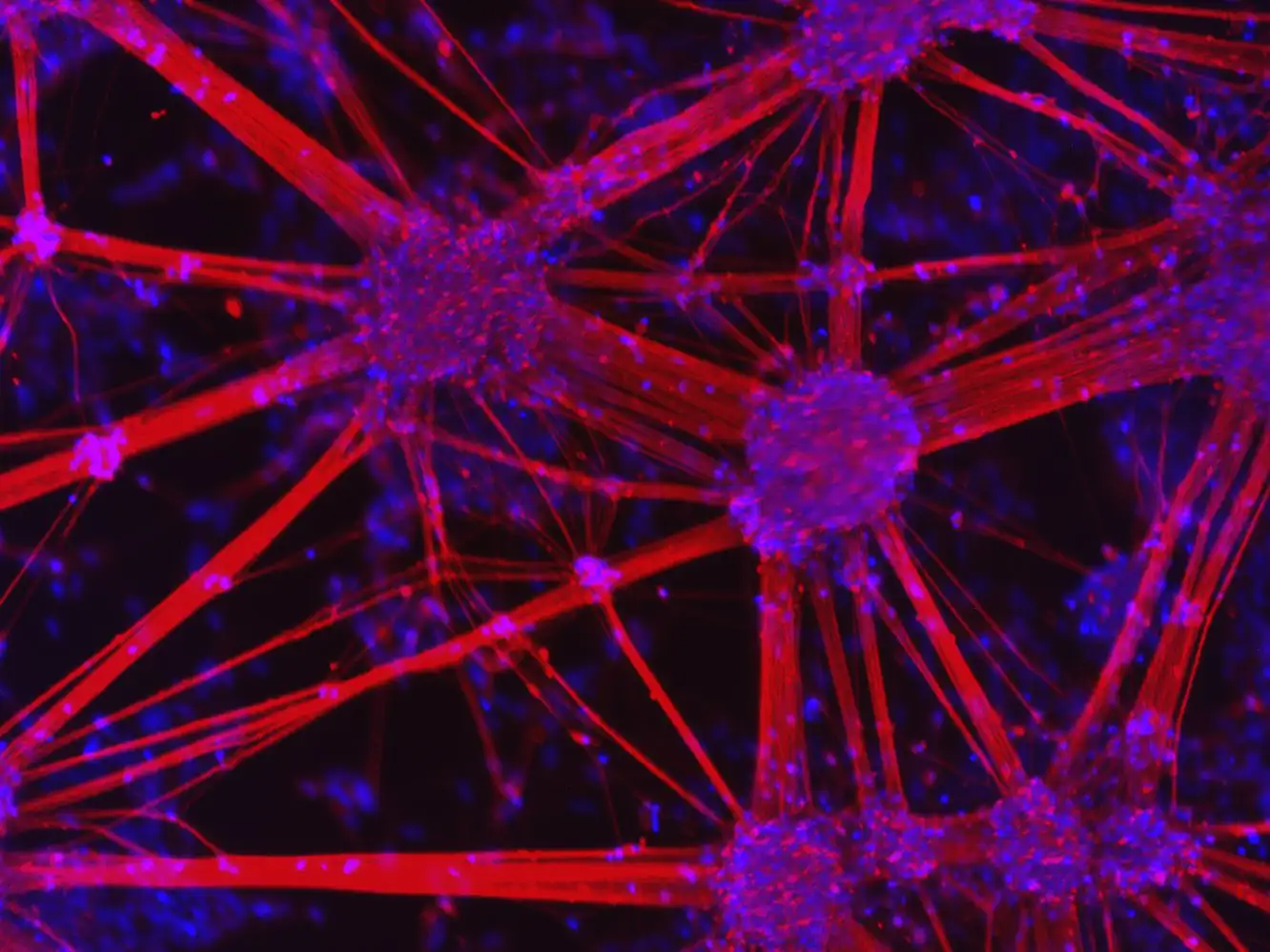
The regulation of movement and cognition by the human brain hinges on two critical types of dopamine-producing neurons, A9 and A10, yet A9 faces a significantly greater risk in Parkinson’s disease.
A9 and A10 dopaminergic neurons originate from the ventral midbrain of the developing brain. As these cells mature, they migrate to distinct regions of the midbrain and express specific genes that define each subtype of dopaminergic neuron. A9 dopaminergic neurons, located in the substantia nigra pars compacta (SNc), project to the dorsolateral striatum, modulating the nigrostriatal dopamine pathway. Meanwhile, A10 dopaminergic neurons, residing in the ventral tegmental area (VTA), project to the nucleus accumbens and prefrontal cortex, regulating the mesolimbic dopamine pathway (Tiklova, Fiorenzano, Grenhoff).
As their name suggests, these cells are responsible for dopamine neurotransmitter release. They also express dopamine autoreceptors which regulate dopamine signaling (Guatteo). A9 dopaminergic neurons primarily govern movement, while A10 dopaminergic neurons contribute to cognition, memory and reward processing (Tiklova). Given their critical roles, these subtypes have been central to neuroscience research for decades. However, due to the limited accessibility of human brain cells, most studies have relied on rodent models.
In recent years, the development of RNA sequencing technologies and advancements in stem cell research have enabled high-resolution analysis of the temporal and spatial genetic profiles of these neurons in the human brain. These breakthroughs have offered deeper insights into their genetic differences, shedding light on the mechanisms underlying Parkinson’s disease.
Vulnerability of A9 Dopaminergic Neurons in Parkinson’s Disease
Parkinson’s disease is a neurodegenerative disorder that is characterized by the progressive loss of dopaminergic neurons, resulting in diminished motor control as well as impairments in cognition and other non-motor functions (Surmeier, Bloem). Among these, A9 dopaminergic neurons are disproportionately affected, with 60–80% of substantia nigra pars compacta (SNc) neurons lost over the course of the disease. By the time initial motor symptoms emerge, 30–50% of these neurons are already degenerated (Cheng). In contrast, A10 dopaminergic neurons, primarily located in the ventral tegmental area (VTA), exhibit greater resistance, with only about 40% lost in PD patients (La Manno).
Although both SNc and VTA dopaminergic neurons originate from the floorplate of the ventral midbrain, gene expression studies reveal significant maturation-driven divergence in genetic profiles, which may explain their distinct functions and their varying vulnerability to Parkinson’s disease. Genes involved in mitochondrial function are particularly relevant, as mitochondrial dysfunction is strongly implicated in Parkinson’s pathology. Studies indicate that mitochondrial dysfunction is more pronounced in the SNc compared to the VTA (Surmeier, Fu).
One key gene in this distinction is OTX2, a mesencephalic factor initially expressed in both subtypes, but retained only in VTA neurons (Panman). OTX2 has been shown to suppress dopamine transporter (DAT) expression, and since high DAT levels correlate with greater vulnerability in Parkinson’s disease, OTX2 can potentially offer neuronal protection to VTA neurons (Fu). Another critical SNc-specific gene, SOX6, is absent in A10 dopaminergic neurons (Panman, Fu), although further research is needed to understand the specific role of SOX6 in A9 dopaminergic neurons.
Beyond genetics, physiological differences also contribute to the heightened vulnerability of A9 neurons. One major factor is their susceptibility to a-synuclein aggregation, a hallmark of Parkinson’s pathology. Accumulation of a-Syn aggregates disproportionately affects SNc neurons, while lower α-Syn levels in other brain regions do not exhibit the same toxic effects (Praschberger, Surmeier).
Interestingly, certain genes expressed in both A9 and A10 neurons regulate each population of cells differently. NURR1, a gene involved in regulation of mitochondrial function, is essential for A9 neuron survival but not for A10 neurons. Notably, as NURR1 expression declines in Parkinson’s disease patients, α-Syn levels increase, potentially exacerbating neurodegeneration (Fu).
Dopaminergic neurons also display a unique slow and broad firing pattern, leading to increased calcium entry through Cav1 calcium channels. Since the calcium buffering protein calbindin (CALB1) is expressed at significantly lower levels in A9 neurons, these cells exhibit higher cytosolic calcium concentrations. This high calcium load induces mitochondrial hyperpolarization, drives reactive oxygen species (ROS) production and amplifies protein damage in mitochondria. All of these factors further compromise neuronal survival (Surmeier).
Unlike A9 neurons, A10 neurons express high levels of CALB1, allowing them to better regulate calcium concentration (Surmeier, Fu). Additionally, studies in rats suggest that CALB1 not only buffers calcium but also plays a role in dopamine release, reinforcing the hypothesis that it has neuroprotective properties in A10 neurons (Fu).
Another vulnerability unique to A9 neurons is their long, unmyelinated axons, which render them more susceptible to mitochondrial dysfunction (Surmeier). Collectively, these genetic and physiological factors contribute to the heightened vulnerability of A9 neurons in Parkinson’s disease, making them a crucial focus for further research.
Pharmacological Effects of Anti-Parkinson’s Disease Drugs on A9 Dopaminergic Neurons
Anti-Parkinson’s disease drugs, primarily dopamine receptor agonists, aim to modulate dopamine release in patients and regulate neuronal excitability. Dopamine itself acts as a key inhibitory modulator, suppressing neuronal firing through potassium channel activation (Guatteo).
Dopaminergic neurons not only release dopamine at their target regions but also within the SNc via regulation of the D2 dopamine autoreceptor. The firing patterns of these neurons, either in regular or bursting mode, influence the amount of dopamine released from the cells. Release of dopamine in the SNc can also cause inhibition of dopamine release at terminal targets (Guatteo, Mercuri).
Electrophysiological Differences Between A9 and A10 Neurons in Dopamine Pathways
Dopaminergic neurons in the nigrostriatal (A9) and mesolimbic (A10) pathways exhibit distinct firing patterns that influence dopamine release. While both neuron populations share fundamental electrophysiological properties, studies indicate subtle yet functionally significant differences between their firing behaviors.
For example, A9 dopaminergic neurons in the SNc exhibit a characteristic pause in firing at the onset of movement, a regulatory mechanism that modulates dopamine concentration in target regions (Dodson). Interestingly, this pause is absent in Parkinson’s disease model mice, suggesting that its disruption may contribute to disease pathology. A10 dopaminergic neurons in the VTA, which govern cognitive and reward processing, follow a different firing pattern, with higher variability in burst activity compared to the more regular bursts seen in A9 neurons (Grenhoff).
Understanding these differences is critical for drug development, as many pharmacological studies rely on rodent models, which do not always accurately reflect human neuron subtype behavior. For instance, A10 neurons used in pharmacological studies often show higher efficacy than A9 neurons (Grenhoff), yet A9 neurons are the primary population affected in Parkinson’s disease. This discrepancy highlights the need for more subtype-specific evaluations when testing dopamine receptor agonists and other neuroprotective therapies.
Additionally, the regulation of dopamine release through potassium channels differs between these two subtypes. Electrophysiological studies show that A9 neurons exhibit stronger responses to AMPK activators, which modulate potassium channel activity, whereas A10 neurons display smaller current shifts (Wu). Moreover, rodent brain slice studies confirm that dopamine receptor agonists, which hyperpolarize neurons to inhibit firing, affect SNc and VTA regions differently (Bowery). These findings underscore the importance of distinguishing nigrostriatal and mesolimbic dopamine regulation, as misinterpreting these physiological nuances could skew drug efficacy predictions.
Conclusion and Perspectives
Decades of research on dopaminergic neurons have deepened our understanding of Parkinson’s disease, revealing key differences in vulnerability between A9 and A10 populations. While animal models have provided valuable insights into the physiological and functional characteristics of these neurons, their limitations underscore the need for more human-specific studies.
Recent advancements in RNA sequencing and stem cell technologies have begun to bridge this gap, enabling more accurate comparisons between rodent and human dopaminergic neurons. Though promising, the available human datasets remain comparatively small, necessitating further investigation into the genetic and physiological mechanisms underlying A9 neuron-specific degeneration in Parkinson’s disease.
Future research efforts must prioritize the development of reliable in vitro human models that accurately mimic A9 dopaminergic neurons in vivo. Recent advancements in induced pluripotent stem cell (iPSC)-derived models, such as TrailBio® A9 Dopaminergic Neurons, are providing an unprecedented opportunity to study these cells with human-specific relevance. By offering a consistent and scalable platform, these products allow researchers to examine disease mechanisms, neuron vulnerability, and drug responses more accurately than traditional models.
As science continues to refine its understanding of A9 neuron vulnerability, targeted interventions may one day slow or even halt the progression of Parkinson’s disease, transforming how we approach neurodegenerative disorders.
References
Surmeier DJ. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018 Oct;285(19):3657-3668. doi: 10.1111/febs.14607. Epub 2018 Aug 14. PMID: 30028088; PMCID: PMC6546423.
Fu Y, Paxinos G, Watson C, Halliday GM. The substantia nigra and ventral tegmental dopaminergic neurons from development to degeneration. J Chem Neuroanat. 2016 Oct;76(Pt B):98-107. doi: 10.1016/j.jchemneu.2016.02.001. Epub 2016 Feb 6. PMID: 26859066.
Mercuri NB, Calabresi P, Bernardi G. The electrophysiological actions of dopamine and dopaminergic drugs on neurons of the substantia nigra pars compacta and ventral tegmental area. Life Sci. 1992;51(10):711-8. doi: 10.1016/0024-3205(92)90479-9. PMID: 1355254.
Grenhoff J, Ugedo L, Svensson TH. Firing patterns of midbrain dopamine neurons: differences between A9 and A10 cells. Acta Physiol Scand. 1988 Sep;134(1):127-32. doi: 10.1111/j.1748-1716.1988.tb08468.x. PMID: 3239415.
Fiorenzano A, Sozzi E, Parmar M, Storm P. Dopamine Neuron Diversity: Recent Advances and Current Challenges in Human Stem Cell Models and Single Cell Sequencing. Cells. 2021 Jun 1;10(6):1366. doi: 10.3390/cells10061366. PMID: 34206038; PMCID: PMC8226961.
La Manno G, Gyllborg D, Codeluppi S, Nishimura K, Salto C, Zeisel A, Borm LE, Stott SRW, Toledo EM, Villaescusa JC, Lönnerberg P, Ryge J, Barker RA, Arenas E, Linnarsson S. Molecular Diversity of Midbrain Development in Mouse, Human, and Stem Cells. Cell. 2016 Oct 6;167(2):566-580.e19. doi: 10.1016/j.cell.2016.09.027. PMID: 27716510; PMCID: PMC5055122.
Tiklová K, Björklund ÅK, Lahti L, Fiorenzano A, Nolbrant S, Gillberg L, Volakakis N, Yokota C, Hilscher MM, Hauling T, Holmström F, Joodmardi E, Nilsson M, Parmar M, Perlmann T. Single-cell RNA sequencing reveals midbrain dopamine neuron diversity emerging during mouse brain development. Nat Commun. 2019 Feb 4;10(1):581. doi: 10.1038/s41467-019-08453-1. PMID: 30718509; PMCID: PMC6362095.
Dodson PD, Dreyer JK, Jennings KA, Syed EC, Wade-Martins R, Cragg SJ, Bolam JP, Magill PJ. Representation of spontaneous movement by dopaminergic neurons is cell-type selective and disrupted in parkinsonism. Proc Natl Acad Sci U S A. 2016 Apr 12;113(15):E2180-8. doi: 10.1073/pnas.1515941113. Epub 2016 Mar 21. PMID: 27001837; PMCID: PMC4839395.
Wu YN, Shen KZ, Johnson SW. Differential actions of AMP kinase on ATP-sensitive K+ currents in ventral tegmental area and substantia nigra zona compacta neurons. Eur J Neurosci. 2017 Dec;46(11):2746-2753. doi: 10.1111/ejn.13756. Epub 2017 Nov 6. PMID: 29057540; PMCID: PMC5716849.
Praschberger R, Kuenen S, Schoovaerts N, Kaempf N, Singh J, Janssens J, Swerts J, Nachman E, Calatayud C, Aerts S, Poovathingal S, Verstreken P. Neuronal identity defines α-synuclein and tau toxicity. Neuron. 2023 May 17;111(10):1577-1590.e11. doi: 10.1016/j.neuron.2023.02.033. Epub 2023 Mar 21. PMID: 36948206; PMCID: PMC10191620.
Bloem BR, Okun MS, Klein C. Parkinson’s disease. Lancet. 2021 Jun 12;397(10291):2284-2303. doi: 10.1016/S0140-6736(21)00218-X. Epub 2021 Apr 10. PMID: 33848468.
Panman L, Papathanou M, Laguna A, Oosterveen T, Volakakis N, Acampora D, Kurtsdotter I, Yoshitake T, Kehr J, Joodmardi E, Muhr J, Simeone A, Ericson J, Perlmann T. Sox6 and Otx2 control the specification of substantia nigra and ventral tegmental area dopamine neurons. Cell Rep. 2014 Aug 21;8(4):1018-25. doi: 10.1016/j.celrep.2014.07.016. Epub 2014 Aug 7. PMID: 25127144.
Guatteo E, Cucchiaroni ML, Sebastianelli L, Bernardi G, Mercuri NB. The midbrain slice preparation. An in vitro model to select potential anti-parkinsonian drugs? Parkinsonism Relat Disord. 2008;14 Suppl 2:S150-3. doi: 10.1016/j.parkreldis.2008.04.020. Epub 2008 Jun 25. PMID: 18583176.
Bowery B, Rothwell LA, Seabrook GR. Comparison between the pharmacology of dopamine receptors mediating the inhibition of cell firing in rat brain slices through the substantia nigra pars compacta and ventral tegmental area. Br J Pharmacol. 1994 Jul;112(3):873-80. doi: 10.1111/j.1476-5381.1994.tb13161.x. PMID: 7921615; PMCID: PMC1910205.
Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010 Jun;67(6):715-25. doi: 10.1002/ana.21995. PMID: 20517933; PMCID: PMC2918373.